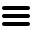CRO Expertise
Gene Therapy Overview
- Housing and breeding programs for transgenic lines
- Experience with administration of various client’s test articles for multiple
gene therapy methods e.g. CRISPR, AAV, Oligo, mRNA, RNAi, siRNA,
ceDNA, antisense - Proficient in multiple routes of administration of test materials for gene therapy
- Multiple tissue collections according to client requests
- Established and developed in-vitro assays to assess the efficiency of the
gene therapy treatments e.g. ammonia, hEPO, FIX, FVIII, PHE, luciferase - Evaluate test compound toxicity by clinical observations and laboratory
test e.g. liver enzymes
IVIS Imaging

Routes of Administration
- Intravenous
- Hydrodynamic intravenous
- Subcutaneous
- Oral gavage
- Intramuscular
- Nebulization
- Intravitreal
- Intrathecal
- Intratracheal
- Intraarticular
- Intranasal
- Intracerebral ventricular
Summary
Experience with multiple transgenic strains
Proficient with multiple dose administration methods
Established various in-vitro assays
IVIS imaging capabilities
White Papers







Webinars
Casey Maguire, Harvard Medical School – Strategies for in vivo Barriers to Gene Therapy Vectors
Technical Sheet
Articles
AAV-S: A versatile capsid variant for transduction of mouse and primate inner ear
Therapeutic efficacy in a hemophilia-B model
JOINN Locations