Our global team of industry-leading scientists employ a variety of state-of-the-art techniques and imaging modalities to investigate the pharmacodynamics (PD), pharmacokinetics (PK) and safety of novel compounds targeted to treat ocular diseases.
Corneal neovascularization, choroidal neovascularization, cataract, glaucoma, and dry eye models are currently available from JOINN (China). Biomere (USA) has a variety of ocular models under development and has interest in collaborative model development on new and unique ocular models. Our combined preclinical and clinical research experience distinctively positions us to support our clients’ nonclinical ophthalmic needs.
| COMPREHENSIVE DOSING TECHNIQUES | SPECIES |
|---|---|
| Topical eye | Mouse, rat, rabbit, NHP |
| Intravitreal injection | Mouse, rat, rabbit, NHP |
| Subretinal injection | Mouse, rat, rabbit, NHP |
| Subconjunctival injection | Mouse, rat, rabbit, NHP |
| Intracameral injection | Mouse, rat, rabbit, NHP |
| Suprachoroidal | Rabbit, NHP |
| Systemic delivery including oral, subcutaneous, intraperitoneal, intrathecal, intratracheal, and intravenous | Mouse, rat, rabbit, NHP |
(CH=China)

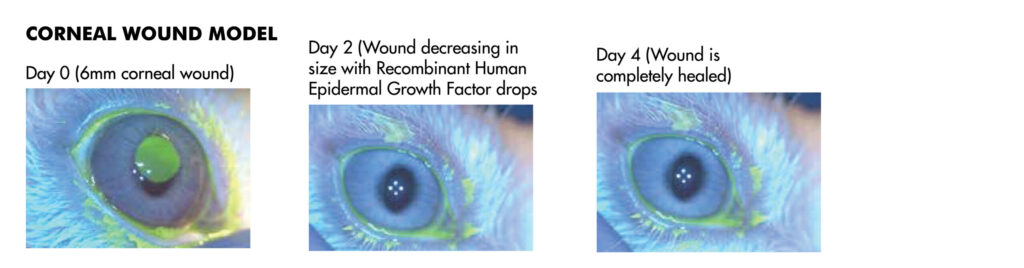
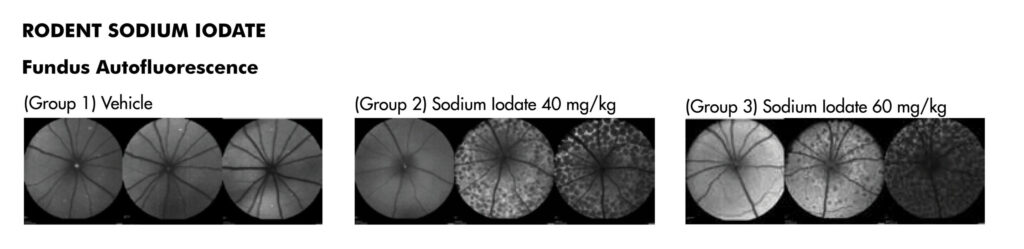
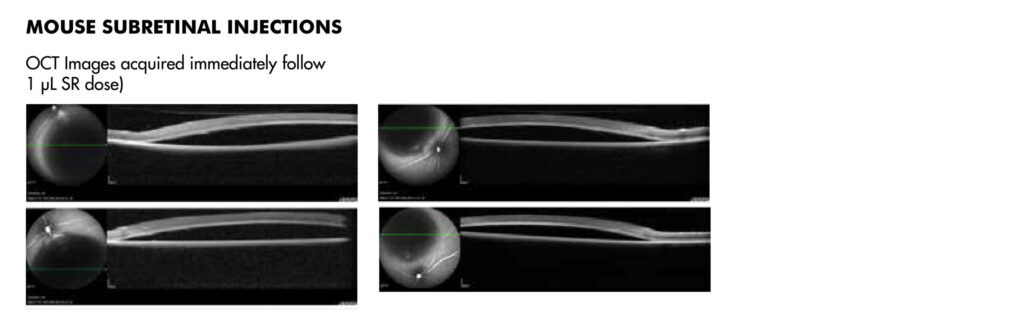
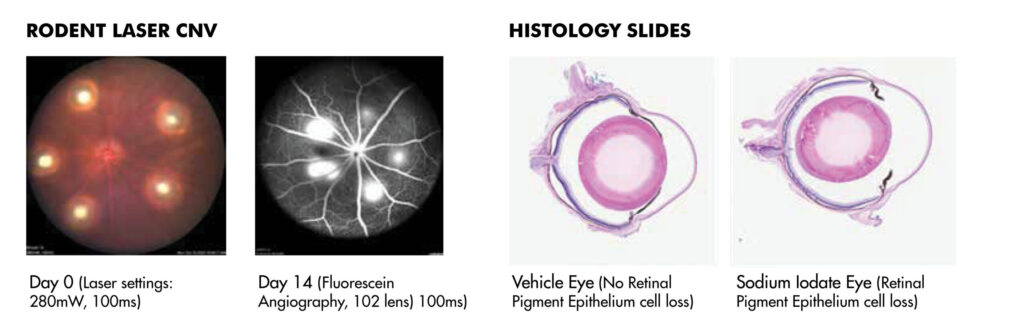
JOINN’s ophthalmology laboratory was established in 2012, in Suzhou. GLP compliant study services are championed by a scientific research team of 35+ professionals. This team is responsible for more than 500 service contracts assisting clients to complete 30 domestic and global IND submissions. Click here to view the JOINN Ocular Photo Gallery.
| ASSESSMENT | SPECIES | EQUIPMENT |
|---|---|---|
| Slit-lamp/anterior segment | Mouse, rat, rabbit, NHP | Kowa handheld sli-lamp, Topcon slit-lamp, leica surgical scope |
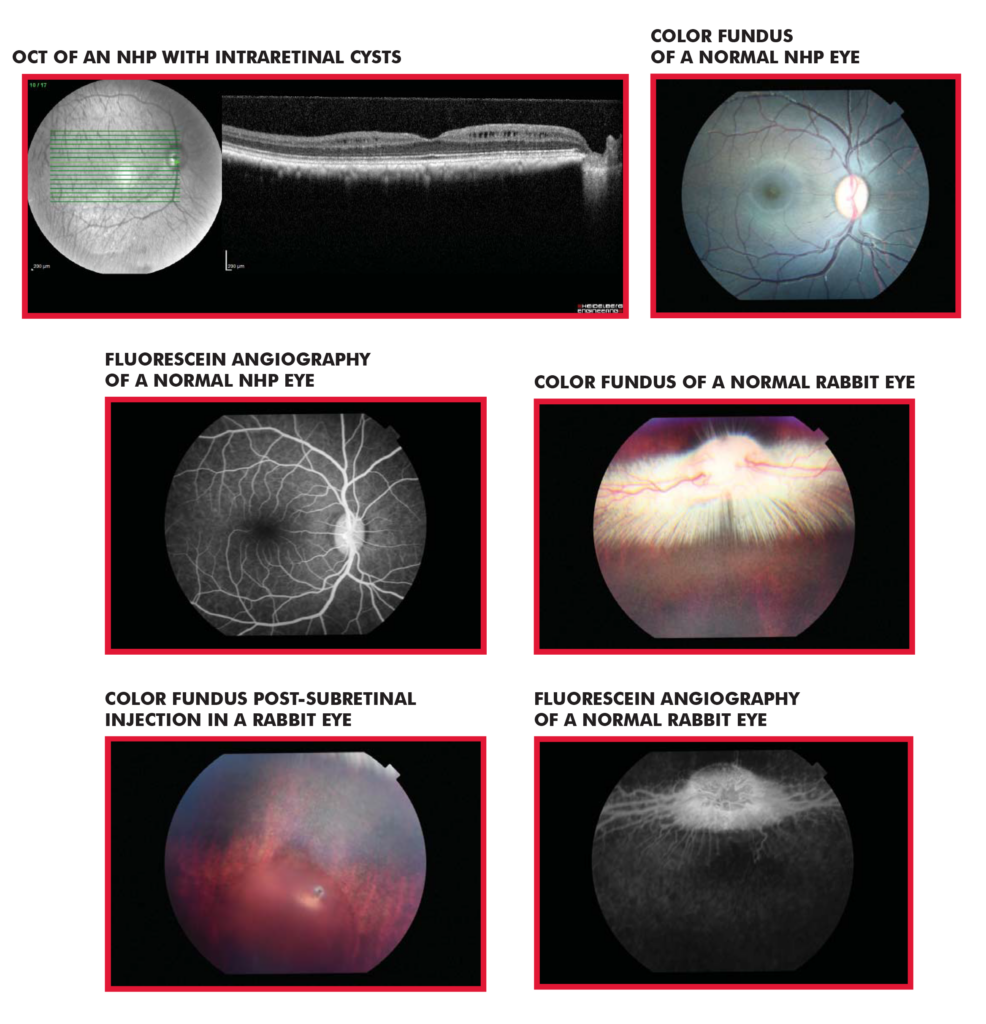
| Indirect ophthalmoscope/fundus | Mouse, rat, rabbit, NHP | Keeler indirect, Volk PictorPlus |
| Optical coherence tomography (OCT) | Mouse, rat, rabbit, NHP | Heidelberg Spectralis OCT +HRA |
| Electroretinogram (ERG) | Rabbit, NHP* | Diagnosys Espion |
| Intra ocular pressure (IOP) | Rabbit, NHP* | Tonovet |
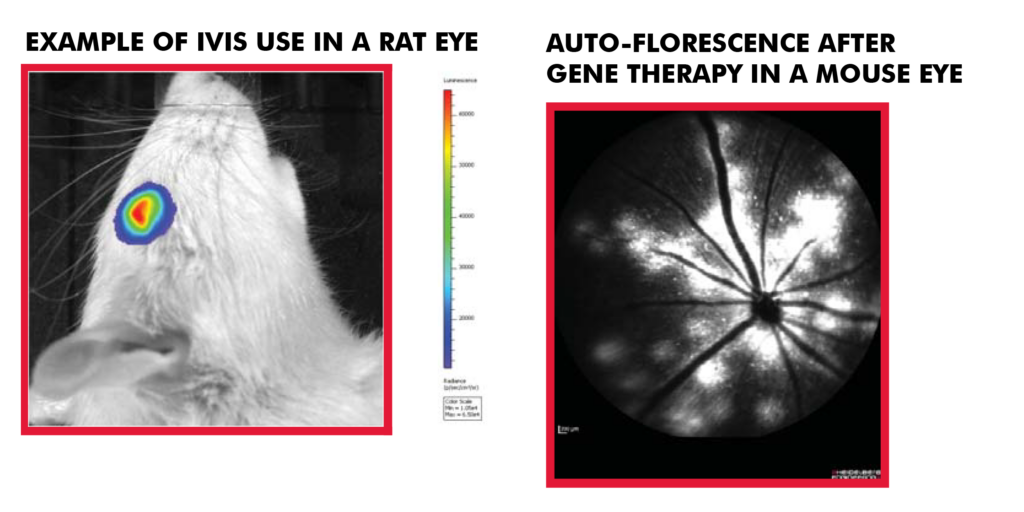
| Gene expression | Mouse and rat, (rabbit and NHP ex vivo) | IVIS Spectrum |
Connect to the Global Liaison Team for more information regarding conducting studies JOINN/China.