Biomere offers rapid, quality-controlled pharmacokinetic (PK) and pharmacodynamic (PD) studies to test your novel compound’s ADME characteristics including biodistribution. Available animal models include: mice, rats, guinea pigs, hamsters, rabbits, and macaques.
Our PK/PD services include testing compounds in Sprague-Dawley rats using LC-MS and histopathology readouts. Plasma samples from rats dosed with 2 doses of dexamethasone were collected at specific time points post administration and analyzed using an Orbitrap Exploris 240 Mass Spectrometer (ThermoFisher Scientific).
Clearance of Dexamethasone from SD rats using LC-MS analysis

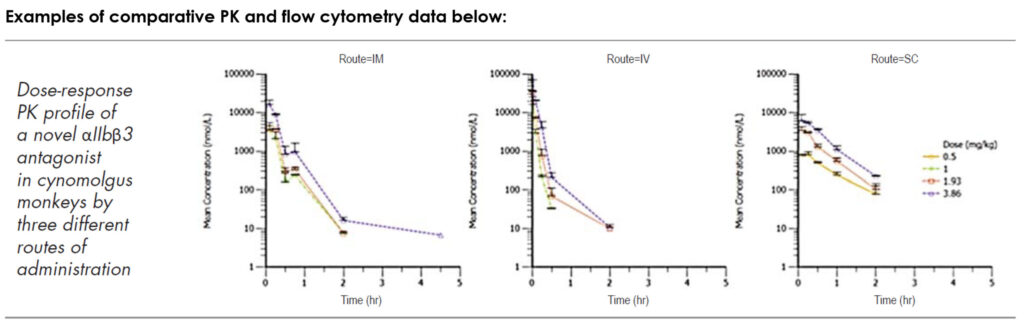
Our NHP program includes a large colony of naïve and non-naïve cynomolgus and rhesus macaques supporting PK/PD discovery research. We utilize a variety of different compound administration and sample collection techniques. Biomere is proficient in conducting xenograft studies, as well as large and varied tissue biopsy and intrathecal programs.