Nonhuman Primate Models for Neurodegenerative Diseases
Chinese Neurodegenerative diseases encompass several disorders that are typically associated with the death of specific neuron types resulting in loss of motor, cognitive and other
Biomere offers rapid, quality-controlled pharmacokinetic (PK) and pharmacodynamic (PD) studies to characterize different therapeutic modalities. We offer multiple species for PK/PD studies, including mice, rats, rabbits and primates. Our team has successfully completed non-GLP PK/PD studies to evaluate multiple modalities, including antibodies, oligos, gene therapies, ocular therapies etc.
Home – PK/PD
Biomere offers comprehensive PK/PD analysis in rodent models (mice and rats) using multiple dosing methods. The most common route of administration is intravenous through the tail vein, but the Biomere team has expertise in retro-orbital IV injection, oral gavage, subcutaneous, intraperitoneal, intramuscular and other routes of administration. Neonate animals can also be dosed using subcutaneous, intraperitoneal and surgical ICV methods. Dosed animals are monitored in-life for body weight changes and clinical observations, which includes blood sample collections. Terminal collections include tissues and blood samples that are shipped to the client or third-party for downstream analysis. Biomere is actively growing the laboratory science portfolio to offer more endpoint assays to clients and partners, with platform providers to offer histopathology and mass spectrometry (LC-MS) analyses.

Our NHP program includes a large colony of naïve and non-naïve cynomolgus macaques supporting PK/PD studies. We offer specialty surgical services, including CSF porting and advanced biopsy techniques, which include liver (open surgery & percutaneous), spleen, muscle and skin. The team offers options for data analysis, such as rapid data sharing, as well as full reporting capabilities. The team has expertise in multiple routes of administration, including:
Different tissues and blood samples can be collected at client-specified timepoints for analysis. Bone marrow aspirates can be collected, as well. At the end of the study, terminal collections of blood and client specified tissue samples.
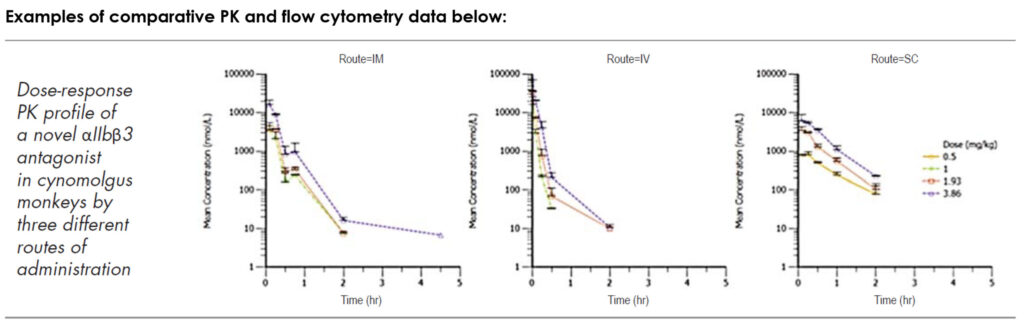
Blood, bone marrow and tissue samples can be collected during or at the end of studies. The samples are frozen or fixed prior to shipment to clients or third-party platform contractors, or can be analyzed at Biomere. Most tissues can be collected, including liver, lymph nodes, skin punches, ocular tissues etc. Blood samples can be analyzed in-house or in collaboration with partners to assess cytokine expression, immunoglobulin (Ig) levels and profiling immune cells using flow cytometry. Additional assays include histopathology, coagulation, chemistry panels and urinalysis. We are expanding our portfolio of laboratory sciences assays to include additional molecular assays. Biomere offers IVIS bioluminescent and fluorescent imaging and ocular-specific OCT and fundus imaging.
Chinese Neurodegenerative diseases encompass several disorders that are typically associated with the death of specific neuron types resulting in loss of motor, cognitive and other
Chinese Nonhuman primates (NHPs) are highly translational models in drug development and are widely used in preclinical efficacy and safety studies1. Due to genotype similarities
Chinese The drug development process has several critical milestones. One of the milestones is pharmacokinetics (PK) studies, which is the study of how a given
Chinese CNS (central nervous system) tumors are primarily located in the brain with some tumors in the spinal cord. CNS tumors can be of various
Chinese Temporal lobe epilepsy (TLE) is a chronic brain disorder where recurrent seizures occur in the temporal lobe. TLE can cause psychological issues, loss of