Evaluate PK/PD, ADME, early toxicology and tolerability of viral vectors and nonviral gene therapies. We have extensive experience in systemic and tissue-specific dosing in small and large animal models.
Home – Gene Therapy
Biomere has developed deep expertise in handling and dosing viral and non-viral gene therapies in small and large animal models. Viral vectors include various serotypes of adeno-associated viruses (AAV), including AAV9 and AAV5. The team can dose lentiviruses in specific primate models (pig-tailed macaques or cynomolgus monkeys). We also have experience with other modalities, including oligonucleotides, RNA-based therapies (mRNA, siRNA), as well as gene editors, including CRISPR and base editing. Biomere has successfully completed several tolerability and PK/PD studies to evaluate nonviral gene delivery systems, such as LNPs (lipid nanoparticles). LNPs have been reported to cause adverse effects, especially at high doses. The Biomere team has the experience of anticipating and counteracting adverse effects, including hematological issues or liver injury.
The Biomere team has expertise in various dosing routes of administration. For rodent models, the most common dosing route is intravenous, while large animals are amenable to multiple dosing routes of administration:
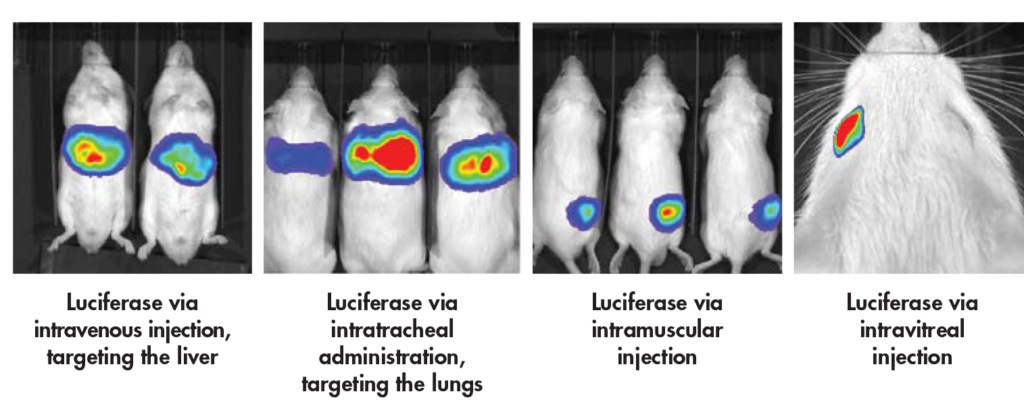
Biomere has experience using transgenic animal models, and the capabilities to house and breed multiple transgenic lines that can be placed on study for ADME programs. The team has expertise in specific disease models, including enzyme deficiency disorders and hematological diseases, such as sickle cell anemia.
Cell and gene therapies typically require detailed preclinical studies to evaluate efficacy, specificity and establish the safety profile. Cell and gene therapies are typically evaluated in animal models that include rodents (mice and rats) and large animals (non-human primates, rabbits and swine). The study objective is critical to determining the model of choice – for example, gene therapies that target the eyes should be evaluated in large animal models, such as rabbits, while initial distribution could be evaluated in mice. Long-term efficacy and safety studies are typically performed in large animal models, such as primates, that have a longer lifespan and have high translational potential to humans.
Preclinical contract research organizations (CROs) support the drug development process to evaluate the efficacy and safety of both viral vectors and nonviral therapies such as LNPs. Evaluating ADME (absorption, distribution, metabolism, excretion) characteristics of gene therapies is essential to understand efficacy and toxicity effects. Some of the key efficacy endpoints include the determination of the therapeutic dose that reaches the target tissue and testing expression of the payload. Another critical endpoint is the measurement of off-target accumulation in other tissues. For example, systemic delivery of an LNP therapy can often lead to accumulation in the liver (liver sink). An important aspect of preclinical evaluation of a gene therapy is understanding metabolism and excretion or clearance. Viral vectors can be shed through biofluids including blood, urine and saliva, which can impact the patient directly or the environment around the patient. An important toxicology endpoint is immunogenicity analysis to evaluate levels of anti-drug antibodies (ADAs) that are also known as neutralizing antibodies.
Chinese The drug development pipeline is increasingly moving towards complex therapies such as monoclonal antibodies and their derivatives, cell and gene therapies and nucleic acid
Chinese Biologics drugs encompass a wide range of therapies including monoclonal antibodies, vaccines, protein and peptide therapeutics, cell therapies, viral vector gene therapies, nucleic acid
CRISPR gene editing has been established as one of the most powerful tools to study disease biology and is also being broadly tested as a
Uveitis is also known as inflammation of the eye where the vascularized middle layer of the eye becomes inflamed resulting in tissue damage and in
CRISPR gene editing is one of the most powerful tools in disease research with the potential to correct genetic defects that cause diseases. CRISPR-mediated gene
RNA-based therapies are a fast-growing class of drugs that utilize the cellular translational machinery and have the potential to change treatment paradigms for several diseases.